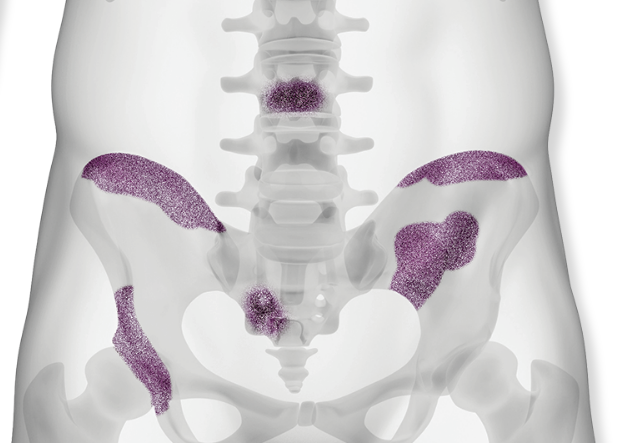
Xofigo® is the only FDA-approved targeted alpha therapy that treats bone metastases in metastatic castration-resistant prostate cancer1
When prostate cancer goes to the bone, so does Xofigo.1
Xofigo can be absorbed by other organs, primarily the bone marrow and digestive system, which may result in side effects in those healthy tissues.
Reference
- Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. Return to content




