About xofigo
SIMPLE DOSING WITH XOFIGO
Xofigo® is conveniently delivered in a patient-ready dose and safely administered in a designated outpatient setting

6 ONE-MINUTE intravenous injections over 5 months.1
There are no post-treatment restrictions, so after treatment patients CAN1:
- Leave the clinic after 1-minute infusion and go about their daily activities
- Safely be in close proximity with, and hug loved ones and children, including being in a car with them
- Share a bed with a partner
- Avoid the need to quarantine
Refer to the full Prescribing Information for specific instructions for patients while receiving Xofigo.
Xofigo is the only radiotherapy to safely ship in a plastic container
Shipping in plastic, Xofigo emits one-fifth of the allowable surface-reading exposure rates: 10 mrem/hr vs an allowable rate of 50 mrem/hra,b
aMax Surface Reading source: www/nrc.gov/docs/ML1124/ML11245A185.pdf.
bShipment of Xofigo – Re-evaluation of shipping parameters; Langhorst, Regists, Siegel; 12 July 2018.
Injections take place every 4 weeks.1
Xofigo is delivered in a convenient, patient-ready dose
- A patient-ready dose with the requested activity will be provided in a syringe by Cardinal Health Nuclear Pharmacy Services2
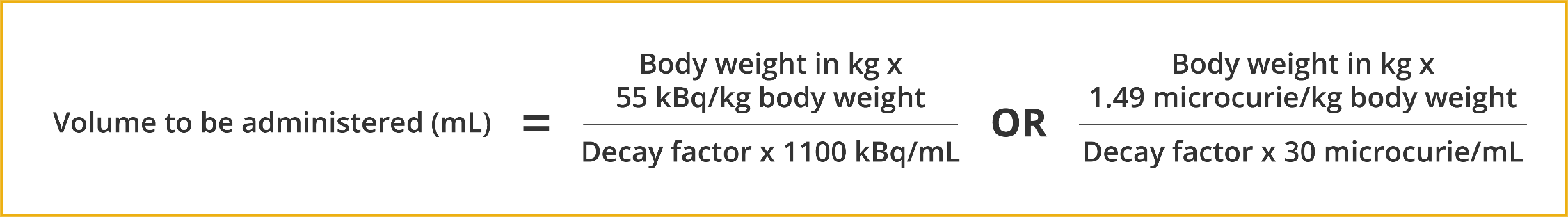
- The patient-ready dose is 1.49 microcurie (55 kBq) per kg body weight1,c
The volume to be administered to a given patient is calculated as follows1,c:
Note: This is for your information only. Cardinal Health will perform all calculations for your convenience.
- The shelf life of Xofigo in the patient-ready syringe is 96 hours3
- Xofigo is a ready-to-use solution and should not be diluted or mixed with any other solutions1
Administration Steps

Assay the dose immediately before treatment, and set up the infusion environment by placing absorbent materials in the area where the injection will take place1

Insert the intravenous (IV) cannula required by your institution for injection. Connect a 2- or 3-way stopcock to the IV device, and then open the stopcock to flush the infusion set with isotonic saline to confirm IV patency1

If IV patency is confirmed, remove the red cap from the Xofigo syringe and attach the open port of the stopcock. Then, turn the stopcock so that it is closed to the saline syringe and open from the Xofigo syringe to the patient1

Administer Xofigo by slow IV injection over 1 minute. When complete, turn the stopcock to shut off the Xofigo syringe and open the saline syringe. Flush the infusion system thoroughly with isotonic saline, remove the syringes and injection setup, and assay immediately for residual activity. Dispose of according to institutional policies1
After administration of Xofigo, the patient should remain in the office for a period of time determined by institutional policies to ensure there are no complications from administration.
Perform standard hematologic evaluations before and during treatment with Xofigo1
Establishing a protocol within your practice for monitoring patients on Xofigo may be beneficial to your patient care.
Before FIRST administration of Xofigo1:
- Confirm
- Absolute neutrophil count (ANC) is ≥1.5 x 109/L
- Platelet count is ≥100 x 109/L
- Hemoglobin count is ≥10 g/dL
Before SUBSEQUENT administrations of Xofigo1:
- Confirm
- ANC is ≥1 x 109/L
- Platelet count is ≥50 x 109/L
If hematologic values do not recover within 6 to 8 weeks after last administration despite supportive care, discontinue further treatment.1
Follow appropriate drug handling1
- Xofigo should be received, used, and administered only by authorized persons in designated clinical settings. The receipt, storage, use, transfer, and disposal of Xofigo are subject to the regulations and/or appropriate licenses of the competent official organization
- Follow normal working procedures for the handling of radiopharmaceuticals, and use universal precautions, such as gloves and barrier gowns, when handling blood and bodily fluids to avoid contamination
- In case of contact with skin or eyes, flush immediately with water
- If spillage occurs, contact the local radiation safety officer immediately to initiate necessary measurements and required procedures to decontaminate
- 0.01 M EDTA solution is recommended to remove contamination
If you have questions, request assistance from a Bayer representative.
EDTA=Ethylene-Diamine-Tetraacetic Acid.
cBased on the 2015 standard set by National Institute of Standards and Technology (NIST), the numerical description of the patient dose has been adjusted from 50 kBq/kg to 55 kBq/kg of body weight and the numerical description of the radioactivity in the vial has been changed from 1000 kBq/mL to 1100 kBq/mL.2
References
- Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. Return to content
- United States Nuclear Regulatory Commission. Revision to the National Institute of Standards and Technology standard for radium-223 and impact on dose calibration for the medical use of radium-223 dichloride. http://pbadupws.nrc.gov/docs/ML1526/ML15264B095.pdf. Accessed April 26, 2022. Return to content
- Data on file. Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. Return to content
Xofigo should be received, used, and administered only by authorized persons in designated clinical settings.
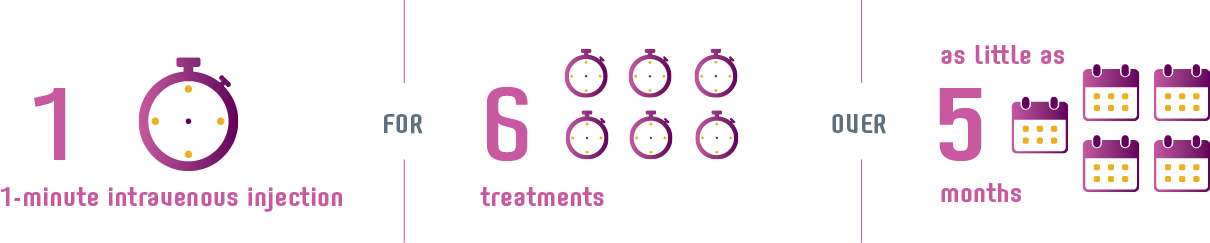
Injections take place every 4 weeks.
XOFIGO IS DELIVERED IN A CONVENIENT, PATIENT-READY DOSE
- A patient-ready dose with the requested activity will be provided in a syringe by Cardinal Health Nuclear Pharmacy Services2
- The patient-ready dose is 1.49 microcurie (55 kBq) per kg body weight1a
The volume to be administered to a given patient is calculated as follows1a:
Note: This is for your information only. Cardinal Health will perform all calculations for your convenience.

- The shelf life of Xofigo in the patient-ready syringe is 96 hours3
- Xofigo is a ready-to-use solution and should not be diluted or mixed with any other solutions1
ADMINISTRATION STEPS

Assay the dose immediately before treatment, and set up the infusion environment by placing absorbent materials in the area where the injection will take place1

Insert the IV cannula required by your institution for injection. Connect a 2- or 3-way stopcock to the IV device, and then open the stopcock to flush the infusion set with isotonic saline to confirm IV patency

If IV patency is confirmed, remove the red cap from the Xofigo syringe and attach the open port of the stopcock. Then, turn the stopcock so that it is closed to the saline syringe and open from the Xofigo syringe to the patient

Administer Xofigo by slow IV injection over 1 minute. When complete, turn the stopcock to shut off the Xofigo syringe and open the saline syringe. Flush the infusion system thoroughly with isotonic saline, remove the syringes and injection setup, and assay immediately for residual activity. Dispose of according to institutional policies1
After administration of Xofigo, the patient should remain in the office for a period of time determined by institutional policies to ensure there are no complications from administration. Fill out the Xofigo Patient Release Card and give it to him before he leaves.
FOLLOW APPROPRIATE DRUG HANDLING1
- Xofigo should be received, used, and administered only by authorized persons in designated clinical settings. The receipt, storage, use, transfer, and disposal of Xofigo are subject to the regulations and/or appropriate licenses of the competent official organization
- Follow normal working procedures for the handling of radiopharmaceuticals, and use universal precautions, such as gloves and barrier gowns, when handling blood and bodily fluids to avoid contamination
- In case of contact with skin or eyes, flush immediately with water
- If spillage occurs, contact the local radiation safety officer immediately to initiate necessary measurements and required procedures to decontaminate
- 0.01 M EDTA solution is recommended to remove contamination
If you have questions, request assistance from a Bayer representative.
EDTA=Ethylene-Diamine-Tetraacetic Acid; IV=Intravenous.
aBased on the 2015 standard set by National Institute of Standards and Technology (NIST), the numerical description of the patient dose has been adjusted from 50 kBq/kg to 55 kBq/kg of body weight and the numerical description of the radioactivity in the vial has been changed from 1000 kBq/mL to 1100 kBq/mL.2
References
- Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. Return to content
- United States Nuclear Regulatory Commission. Revision to the National Institute of Standards and Technology standard for radium-223 and impact on dose calibration for the medical use of radium-223 dichloride. http://pbadupws.nrc.gov/docs/ML1526/ML15264B095.pdf. Accessed March 11, 2016. Return to content
- Data on file. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; 2016. Return to content


