ACCESS
CODING AND BILLING
Please read the following information to help facilitate patient access, successful claims filing, and accurate reimbursement for Xofigo®
- Coding and billing varies by site of care:
- Freestanding centers of care use the CMS-1500 (version 02-12) claim form
- Hospital outpatient sites of care use the UB-04/CMS-1450 claim form
- Accurate completion of the Freestanding Center (CMS-1500) or Hospital Outpatient (UB-04/CMS-1450) claim form is important for timely and appropriate claims processing and to avoid claim denials
- Be familiar with Medicare and private payor coding and billing requirements
- Understand and document on claims the payor’s clinical criteria for determining which patients are medically appropriate for Xofigo
- Accurately code the patient’s diagnosis and service for appropriate claims processing and reimbursement
Coding and billing support for Xofigo
Xofigo® Access Services can provide information on payor coding, billing, and claims submission requirements to help you with claims completion.
Healthcare professionals administering Xofigo in a freestanding center should submit a CMS-1500 form or its electronic equivalent to report the use and administration of Xofigo.
Guidance on filling out the CMS-1500 Claim Form (version 02-12 as of April 2014):

aOther diagnosis codes may be applicable; code(s) and sequencing order may vary by payor.
Xofigo and the associated services provided in a hospital outpatient setting are billed on the UB-04 claim form or its electronic equivalent.
Guidance on filling out the UB-04 claim form:

aOther diagnosis codes may be applicable; code(s) and sequencing order may vary by payor.
Billing for Xofigo and its administration in separate sites of carea,b
According to Medicare guidance, if a physician who is not employed by the hospital administers Xofigo to a patient in that hospital’s outpatient department, and Xofigo was purchased by the hospital, the hospital outpatient department may submit a separate claim for the product Xofigo and the technical component of its administration.
Guidance on filling out the UB-04 claim form when billing separate sites of care:

aThe technical component includes nonphysical work, including administrative, personnel, equipment, and facility costs and related malpractice expenses.
bOther diagnosis codes may be applicable; code(s) and sequencing order may vary by payor.
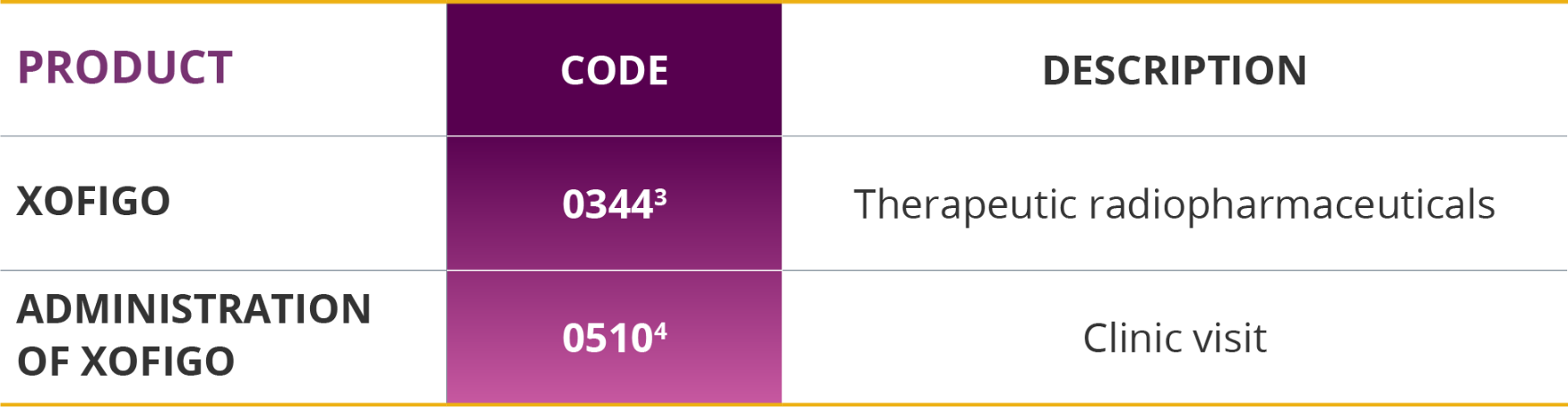
Xofigo and its associated services may be reported with the codes listed below.
Healthcare Common Procedure Coding System (HCPCS) codes
In a freestanding center or hospital outpatient setting, Xofigo is reported using the product-specific HCPCS A-code, A9606 (radium Ra 223 dichloride, therapeutic, per microcurie).1

Please note that individual Medicare Administrative Contractors (MACs), private payors, or other payors or claims processors may have different coding requirements for radiopharmaceuticals in the freestanding center. Xofigo Access Services can research payor-specific coding requirements in performing patient-specific benefit verifications.
Providers should confirm the appropriate coverage, coding, and reimbursement with the applicable payor or claims processor before submitting claims for an item or service. Providers must ensure that all claims submitted to payors are accurate, complete, and adequately supported by documentation in the medical record.
Payors differ on guidelines and criteria required for billing an office visit on the same day as other physician services. It is important to verify appropriate coding with a patient’s health insurance plan before submitting the CMS-1500 claim form for reimbursement. Additional information required by the payor may include:
- Xofigo Prescribing Information
- FDA approval letter for Xofigo
- Patient medical history
- Physician clinical notes on the patient’s condition
- Letter of medical necessity
- Invoice for Xofigo
- National Drug Code for Xofigo (Medicaid and/or commercial payors)
Current Procedural Terminology (CPT) codes
Physicians use CPT codes to report medical services provided in a freestanding center or hospital outpatient setting, including the administration of Xofigo.

Revenue codes for the UB-04 claim form
The UB-04 claim form also requires documentation of revenue codes associated with services provided to patients receiving Xofigo. Please confirm payor guidelines as other revenue codes, including 0636 (drugs requiring detailed coding), may also be appropriate when submitting a claim for Xofigo.
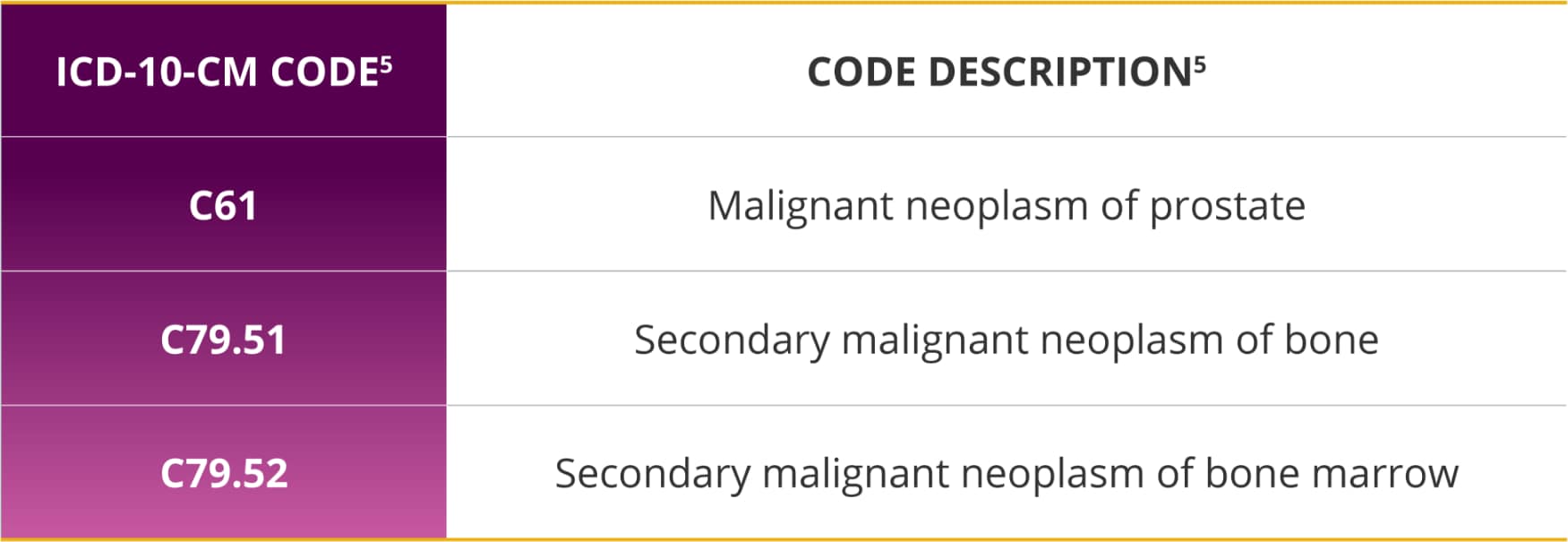
International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10 CM) codes (as of October 1, 2015)
Appropriately coding and classifying the patient’s diagnosis and condition is important to support medical necessity for receiving Xofigo.
Xofigo is indicated for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bone metastases and no known visceral metastatic disease.
Evaluation and management (E/M) codes may also be used to describe services provided by the physician when the patient’s condition is significant and beyond the intravenous injection of Xofigo. If an E/M service is billed in addition to the intravenous injection of Xofigo, the modifier “-25” is necessary to indicate a significant and separately identifiable E/M service by the same physician on the same day. The provider must document the additional service in the patient’s medical record.
aInformation provided on this page is for informational purposes only and does not guarantee that codes will be appropriate or that coverage and reimbursement will result. Customers should consult with their payors for all relevant coverage, coding, and reimbursement requirements. It is the sole responsibility of the provider to select proper codes and ensure the accuracy of all claims used in seeking reimbursement. Neither this resource nor Xofigo Access Services is intended as legal advice or as a substitute for a provider’s independent professional judgment.
It is important for prescribing physicians to understand payor policies and requirements affecting patient access and product coverage, coding, and reimbursement. Conduct an insurance benefit verification for your patient prior to treatment with Xofigo to identify the necessary requirements.
MEDICARE
- Medicare coverage of Xofigo may be available to beneficiaries if reasonable and necessary
- Options for supplemental insurance coverage for patients already enrolled in traditional Medicare include Medigap plans or a supplemental commercial insurance plan
- A Medigap plan may cover a patient’s 20% coinsurance remaining after Medicare pays 80%
- Third-party financial assistance options may be available for eligible patients in need
- Medicare Administration Contractors (MACs) have different fee schedules for physician-owned practices
- Managed Medicare Plans do not publish fee schedules so check your fee before testing
MEDICAID
- Medicaid coverage and reimbursement policies may vary state to state and depend on individual insurance plans and negotiated contracts with physicians and hospitals
- State Medicaid agencies may require additional documentation to grant coverage or obtain prior authorization for Xofigo
- Patient cost-share will vary based on the state Medicaid policies, but may require patients to pay a minimal copay or coinsurance for Xofigo
- Third-party financial assistance options may be available for eligible patients in need
COMMERCIAL PAYORS
- Commercial payors develop their own coverage and reimbursement policies and depend on individual insurance plans and negotiated contracts with physicians and hospitals
- Contracted prices may be based on fee schedules, invoices, or other payment methodologies such as average selling prices
- Sites should review their payor contracts to determine specific payment methodology
- Commercial payors may also require additional documentation to grant coverage or obtain prior authorization for Xofigo
- Patients may be responsible for a copay and deductible based on their individual insurance plan
- Please consult local payors for specific claims-submission guidance
- Financial assistance options are available for eligible patients in need
- Managed Commercial Plans do not publish fee schedules so check your fee before testing
Learn about copay assistance with Xofigo
CLAIMS PROCESSING
Providing documentation will support payors in processing a claim for Xofigo.
- FDA approval letter for Xofigo
- Patient medical history
- Physician’s clinical notes on the patient’s condition
- Invoice for Xofigo
In the event the payor made an error in processing the claim, the administering provider’s office should contact the payor to discuss the processing issue in order to allow the patient access to Xofigo.
CLAIMS APPEAL
When appealing a denied claim, payors may request additional documentation (if not submitted with the original claim), including:
- Sample letter of appeal
- Copy of the original claim
- Copy of denial notification from payor
- Xofigo Prescribing Information
- FDA approval letter
- Copy of invoice
- Patient medical history, including clinical notes
- Copy of referring physician’s order
aInformation provided is for informational purposes only and does not guarantee that codes will be appropriate or that coverage and reimbursement will result. Customers should consult with their payors for all relevant coverage, coding, and reimbursement requirements. It is the sole responsibility of the provider to select proper codes and ensure the accuracy of all claims used in seeking reimbursement. Neither this resource nor Xofigo® Access Services is intended as legal advice or as a substitute for a provider’s independent professional judgment.
Click here to contact your Bayer Field Reimbursement Manager to discuss further.
Choose your state and select a site of care for coverage, coding, billing/claims submissions, and payment-methodology information from the MACs.a

aInformation provided is for informational purposes only and does not guarantee that codes will be appropriate or that coverage and reimbursement will result. Customers should consult with their payors for all relevant coverage, coding, and reimbursement requirements. It is the sole responsibility of the provider to select proper codes and ensure the accuracy of all claims used in seeking reimbursement. Neither this resource nor Xofigo® Access Services is intended as legal advice or as a substitute for a provider’s independent professional judgment.
Click here to contact your Bayer Field Reimbursement Manager to discuss further.
Comprehensive support right when you need it

Counselors are available from 9:00 AM to 7:00 PM ET, Monday through Friday:
- Call 1-855-6XOFIGO (1-855-696-3446)
- Fax 1-855-963-4463
- Visit XofigoAccessOnline.com
For helpful resources, click here.
References
- Centers for Medicare & Medicaid Services. HCPCS release and code sets. Alpha-numeric HCPCS items. 2020. https://www.cms.gov/medicarecodinghcpcsreleasecodesetsalpha-numeric-hcpcs/2020. Accessed May 5, 2022. Return to content
- American Medical Association. 2012 CPT Professional Edition. American Medical Association; 2011. Return to content
- Centers for Medicare & Medicaid Services. CMS Manual System. Pub. 100-04 Medicare Claims Processing. Transmittal 423. 2005. http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R423CP.pdf. Accessed May 5, 2022. Return to content
- Centers for Medicare & Medicaid Services. CMS Manual System. Pub. 100-04 Medicare Claims Processing. Transmittal 167. 2004. http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R167CP.pdf. Accessed May 5, 2022. Return to content
- Ingenix. 2014 International Classification of Diseases, 10th Revision, Clinical Modification Mappings. OPTUMInsight, Inc.; 2013. Return to content
Please consider the following information to help facilitate patient access, successful claims filing, and accurate reimbursement for Xofigo
- Coding and billing varies by site of care:
- Accurate completion of the Freestanding Center (CMS-1500) or Hospital Outpatient (UB-04/CMS-1450) claim form is important for timely and appropriate claims processing and to avoid claim denials
- Be familiar with Medicare and private payor coding and billing requirements
- Understand and document on claims the payor’s clinical criteria for determining which patients are medically appropriate for Xofigo
- Accurately code the patient’s diagnosis and service for appropriate claims processing and reimbursement
Coding and billing support for Xofigo
Xofigo® Access Services can provide information on payor coding, billing, and claims submission requirements to help you with claims completion.
Consult with your Field Reimbursement Manager for specific coding and billing guidance.
Comprehensive support right when you need it

Counselors are available from 9:00 AM to 7:00 PM ET, Monday through Friday:
- Call 1-855-6XOFIGO (1-855-696-3446)
- Fax 1-855-963-4463
- Visit XofigoAccessOnline.com
aInformation provided on this page is for informational purposes only and does not guarantee that codes will be appropriate or that coverage and reimbursement will result. Customers should consult with their payors for all relevant coverage, coding, and reimbursement requirements. It is the sole responsibility of the provider to select proper codes and ensure the accuracy of all claims used in seeking reimbursement. Neither this resource nor Xofigo Access Services is intended as legal advice or as a substitute for a provider’s independent professional judgment.

