EFFICACY
OVERALL SURVIVAL (OS)
Xofigo® extended OS by 3.6 months (14.9 months vs 11.3 months) in exploratory analysis1,2
PRESPECIFIED INTERIM OS ANALYSIS
- Median OS was 14.0 months (95% CI: 12.1-15.8) for Xofigo + best standard of care (BSOC) vs 11.2 months for BSOC (95% CI: 9.0-13.2). Hazard ratio (HR)=0.695 (95% CI: 0.552-0.875) P=0.001851
- Evaluated in the ALSYMPCA trial: double-blind, randomized, placebo-controlled, phase III study of 921 patients with castration-resistant prostate cancer with symptomatic bone metastases and no known visceral metastatic disease1,2
- At the preplanned interim analysis, 809 patients were randomized to receive Xofigo 55 kBq (1.49 microcurie)/kg intravenously every 4 weeks for 6 cycles (n=541) + BSOC or BSOC (n=268); statistically significant improvement seen in interim analysis1
- An exploratory updated analysis was performed before patient crossover, incorporating an additional 214 events, resulting in findings consistent with the interim analysis1
- BSOC was defined as antiandrogens, local external-beam radiation therapy, ketoconazole, estrogens, estramustine, or treatment with glucocorticoids2
EXPLORATORY UPDATED OS ANALYSIS
- Median OS was 14.9 months (95% CI: 13.9-16.1) for Xofigo + BSOC vs 11.3 months for BSOC (95% CI: 10.4-12.8). HR=0.695 (95% CI: 0.581-0.832)1,2
MEDIAN OS IN AN UPDATED EXPLORATORY ANALYSIS1,2
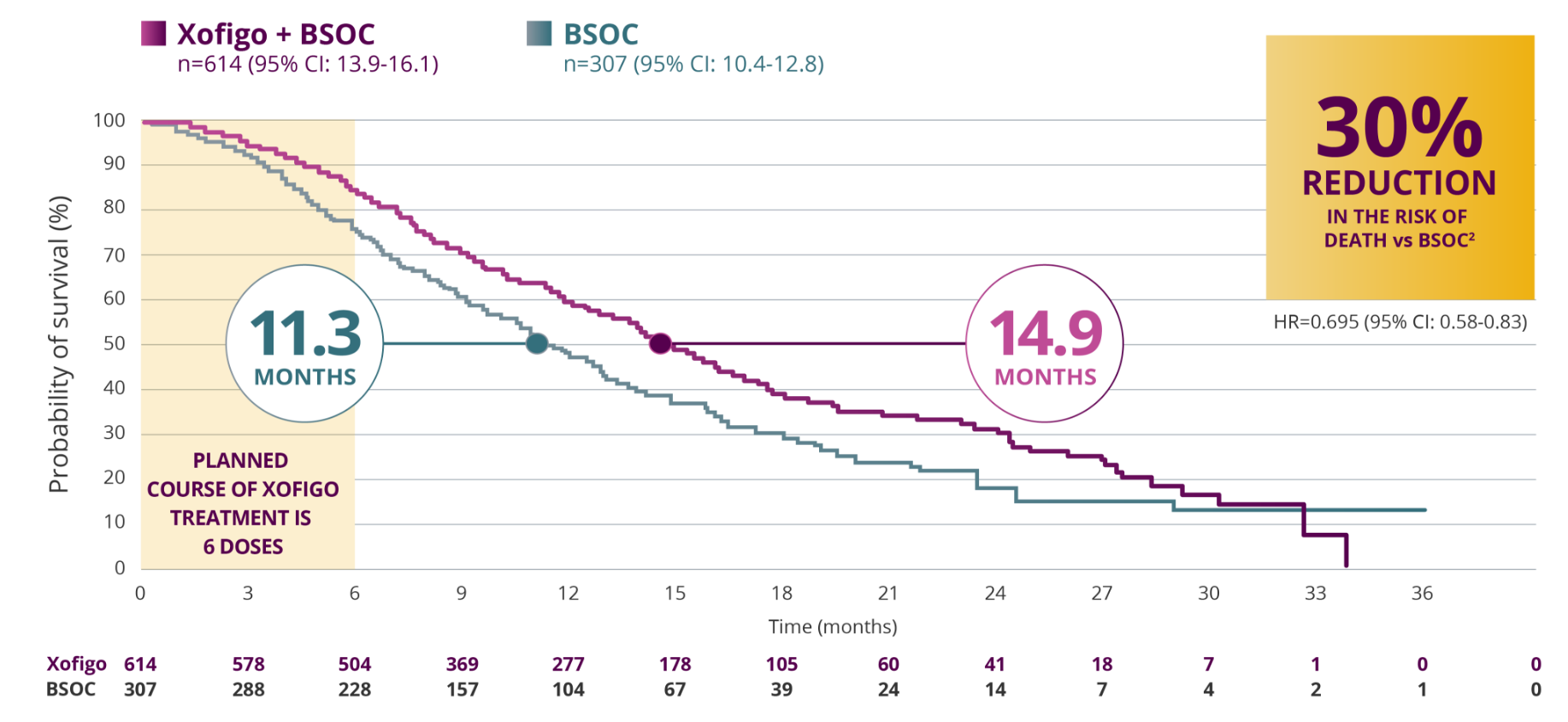
CI=Confidence Interval.
Give your patients the survival benefit with 6 injections of Xofigo: a 1-minute injection every 4 weeks
OS ANALYSES BY PRESPECIFIED SUBGROUPS1
PRESPECIFIED SUBGROUP SURVIVAL ANALYSIS FROM ALSYMPCA1
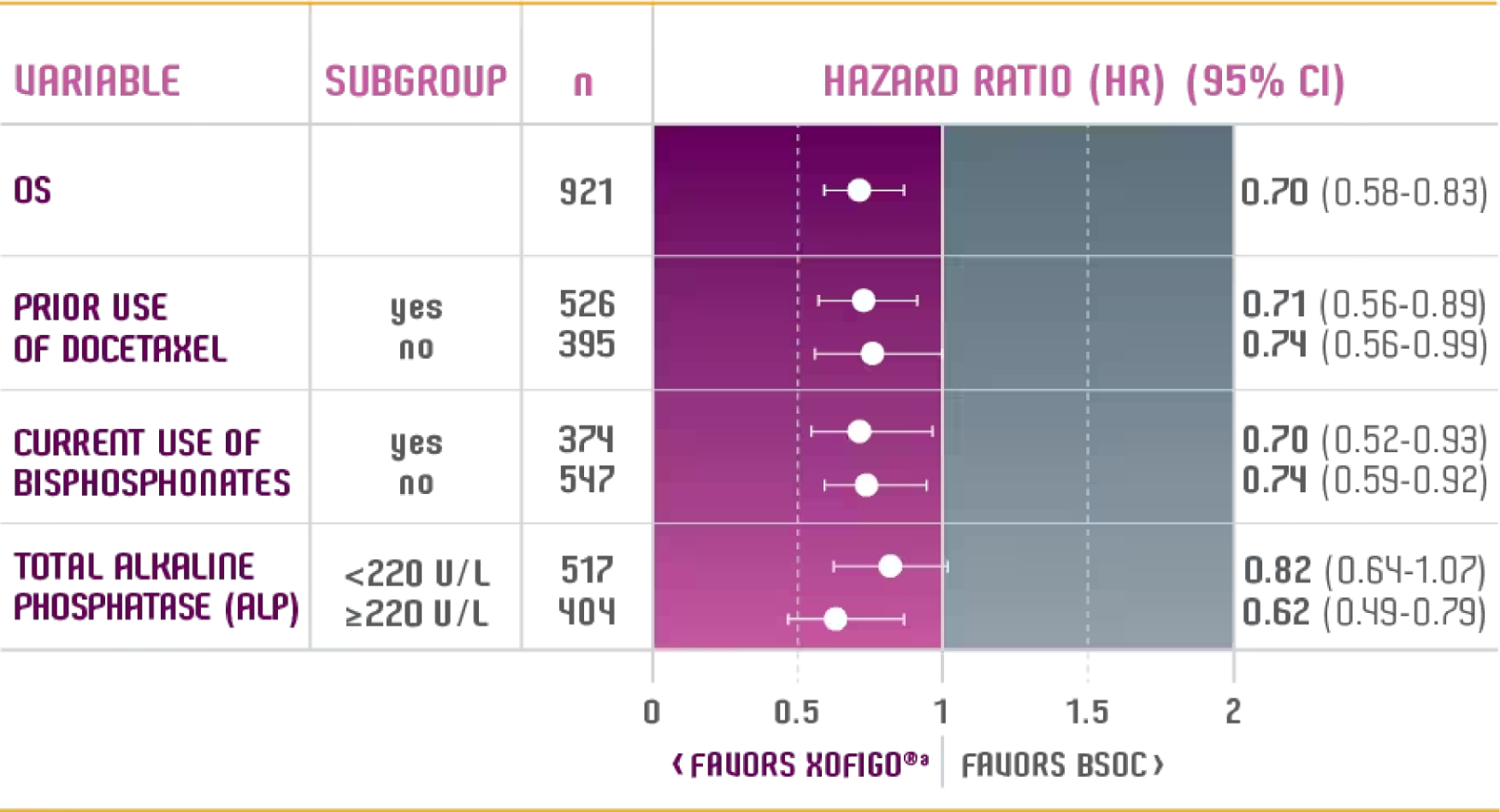
Adapted from Parker, et al.
- The primary endpoint of the ALSYMPCA study was OS2
- Patients were stratified into the following subgroups at randomization: prior docetaxel exposure, current bisphosphonate use, and total ALP2
- These subgroup analysis data are descriptive in nature—the study was not powered to detect treatment differences in OS specifically within these prestratified subgroups
aPlus best standard of care (BSOC).
PSA is not a biomarker for Xofigo therapy progression PSA=Prostate-Specific Antigen.
References
- Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. Return to content
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. Return to content

