MYTHS
FACTS
PRESPECIFIED INTERIM OS ANALYSIS:
- Median OS was 14.0 months (95% CI: 12.1-15.8) for XOFIGO + best standard of care (BSOC) vs 11.2 months for BSOC (95% CI: 9.0-13.2).
Hazard ratio (HR)=0.695 (95% CI: 0.552-0.875) P=0.001851 - Evaluated in the ALSYMPCA trial: double-blind, randomized, placebo-controlled, phase III study of 921 patients with castration-resistant prostate cancer with symptomatic bone metastases and no known visceral metastatic disease1,2
- At the preplanned interim analysis, 809 patients were randomized to receive XOFIGO 55 kBq (1.49 microcurie)/kg intravenously every 4 weeks for 6 cycles (n=541) + BSOC or BSOC (n=268); statistically significant improvement was seen in the interim analysis1
- An exploratory updated analysis was performed before patient crossover, incorporating an additional 214 events, resulting in findings consistent with the interim analysis1
- BSOC was defined as antiandrogens, local external-beam radiation therapy, ketoconazole, estrogens, estramustine, or treatment with glucocorticoids2
EXPLORATORY UPDATED OS ANALYSIS:
- Median OS was 14.9 months (95% CI: 13.9-16.1) for XOFIGO + BSOC vs 11.3 months for BSOC (95% CI: 10.4-12.8). HR=0.695 (95% CI: 0.581-0.832)1,2
MEDIAN OS IN AN UPDATED EXPLORATORY ANALYSIS1,2
References: 1. Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. 2. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
XOFIGO CAN BE AN OPTION ACROSS MULTIPLE SCANNING MODALITIES1,3,4
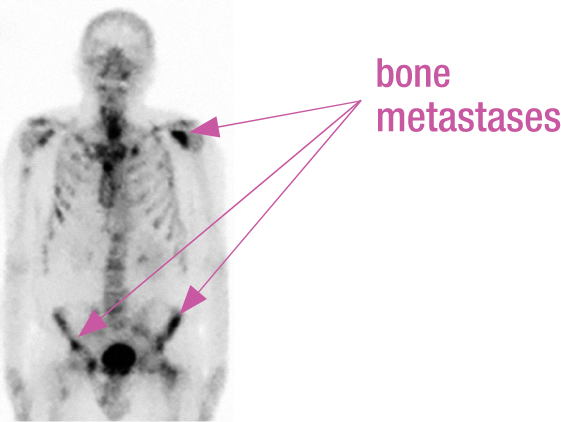
BONE SCAN
Bone scintigraphy showed diffuse bone metastases in pelvic, spinal, and rib region.
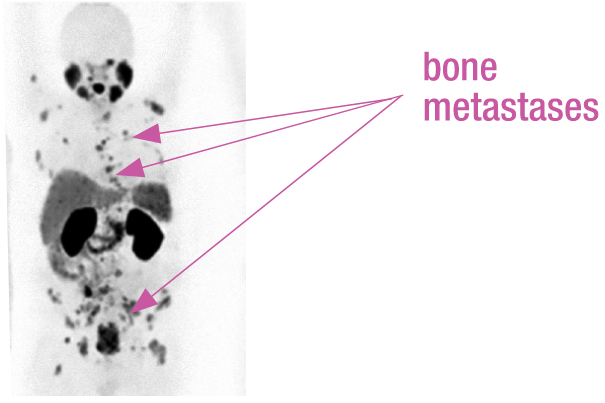
PSMA-PET SCAN
68Ga-labelled PSMA-PET/CT showed diffuse bone metastases in pelvic, spinal, and rib region, but no visceral involvement.
Real patient case from published literature: A 69-year-old patient with hormone-refractory prostate cancer (Gleason score, 8) was referred for XOFIGO. PSA level at time of PET imaging was 30 ng/mL, and ALP was 207 U/L.
IN mCRPC XOFIGO CAN BE USED REGARDLESS OF PSMA STATUS2
XOFIGO is indicated for the treatment of patients with CRPC symptomatic bone metastases, and no known visceral metastatic disease.1
ALP=alkaline phosphatase; ARi=androgen receptor inhibitor; CI=confidence interval; CT=computed tomography; EBRT=external-beam radiation therapy; mCRPC=metastatic castration-resistant prostate cancer; PSA=prostate-specific antigen; PSMA=prostate-specific membrane antigen; PSMA-PET=prostate-specific membrane antigen positron emission tomography.
References: 1. Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. 2. De Vincentis G, Gerritsen W, Gschwend JE, et al. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019;30(11):1728-1739. 3. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. 4. Ahmadzadehfar H, Azgomi K, Hauser S, et al. Ga-PSMA-11 PET as a gatekeeper for the treatment of metastatic prostate cancer with 223Ra: proof of concept. J Nucl Med. 2017;58(3):438-444.
ALSYMPCA SUBGROUP ANALYSIS1,a
MEDIAN INCREASE IN OS IN CHEMOTHERAPY-NAÏVE PATIENTS
aChemotherapy-naïve patients from ALSYMPCA were defined as docetaxel naÏve given study criteria.
- In chemotherapy-experienced patients, median increase in OS with XOFIGO (n=352) vs BSOC (n=174) was 3.1 months (14.4 months vs 11.3 months. HR=0.71 [95% CI: 0.56-0.89; n=526])1
- The primary endpoint of the ALSYMPCA study was OS1
- Patients were stratified into the following subgroups at randomization: prior docetaxel exposure, current bisphosphonate use, and total alkaline phosphatase1
- These Post hoc, Exploratory subgroup analysis data are descriptive in nature—the study was not powered to detect treatment differences in OS specifically within these prestratified subgroups1
PERCENTAGE OF PATIENTS WITH HEMATOLOGIC LABORATORY VALUES CORRESPONDING TO GRADE 3-4 ADVERSE EVENTS (AEs) IN THE CHEMOTHERAPY POST-STUDY DRUG GROUP2
STUDY BACKGROUND
- An exploratory analysis of prospectively collected data (from the ALSYMPCA patient subgroup who received chemotherapy after completing their assigned study drug treatment) was conducted to evaluate the safety of chemotherapy following XOFIGO2
OVERALL RESULTS
- Patients in the XOFIGO group had a longer time from randomization to the start of chemotherapy vs placebo (median 9.1 months vs 7.5 months, respectively); median duration of first chemotherapy was 4.6 months for XOFIGO vs 4.2 months for placebo2
bLast nonmissing measurement prior to start of first post-study drug chemotherapy. Timing of hematology laboratory values is determined according to start of chemotherapy, not by protocol-defined visits.2
GRADE 3-4 AEs IN PATIENTS RECEIVING CHEMOTHERAPY WERE SIMILAR REGARDLESS OF PRIOR XOFIGO USE2
- Incidence of Grade 3-4 hematologic AEs from baseline up to 18 months following first post-study drug chemotherapy was generally low (≤10%), but tended to be more common among patients in the XOFIGO group2
- Grade 3-4 AEs for hemoglobin, neutrophils, and platelets were recorded in 8%, 10%, and 6% of XOFIGO and 4%, 2%, and 2% of placebo patients, respectively2
- At months 6 and 12 of the trial, 1 patient presented with elevated neutropenia, and 1 patient presented with anemia2
AE=adverse event; BSOC=best standard of care; HR=hazard ratio; OS=overall survival.
References: 1. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. 2. Sartor O, Hoskin P, Coleman RE, et al. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate. 2016;76(10):905-916.
WELL-DOCUMENTED SAFETY PROFILE: OVERALL GRADE 3-4 ADVERSE EVENTS (AEs) WERE LOWER THAN PLACEBO2
Adverse Reactions Occurring in ≥2% of Patients Who Received Concomitant EBRT in the Randomized Trial2,a,b
Based on post hoc analysis of the ALSYMPCA trial. These results are considered exploratory. During the study, 30% (186/614) of XOFIGO patients and 34% (105/307) of placebo patients received concomitant EBRT for bone pain.1
- At any time prior to randomization, 50% (306/614) of XOFIGO patients and 49% (149/307) of placebo patients received EBRT to bone1
- Within 12 weeks prior to randomization, 16% (99/614) of XOFIGO patients and 16% (48/307) of placebo patients received EBRT to bone1
- The most common hematologic laboratory abnormalities in the XOFIGO arm (≥10%) vs the placebo arm (all grades [%]), respectively, were anemia (93% vs 88%), lymphocytopenia (72% vs 53%), leukopenia (35% vs 10%), thrombocytopenia (31% vs 22%), and neutropenia (18% vs 5%)2
EBRT=external-beam radiation therapy.
Patients were randomized 2:1, XOFIGO:placebo.2
aFor which the rates for Xofigo exceed the rates for placebo.2
bAEs per NCI-CTCAE v3.0.3
cPlus best standard of care.2
References: 1. Finkelstein SE et al. Presented at: 2015 Annual ASCO Meeting; May 29-June 2, 2015; Chicago, Illinois. Abstract 182. 2. Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. 3. Data on file. Bayer HealthCare Pharmaceuticals Inc. Whippany, NJ.
- RaLu is a retrospective medical chart review of 133 patients from Nuclear Medical centers who received XOFIGO → Taxane → 177Lu-PSMA vs Taxane → XOFIGO → 177Lu-PSMA therapies2
- 13 patients who received taxane both before and after XOFIGO were included in both groups2
- 71% received 6 XOFIGO injections2
- 56% of patients received ≥4 life-prolonging therapies, including abiraterone (71%), enzalutamide (70%), docetaxel (74%) before starting 177Lu-PSMA2
- 73% of patients received 1–4 177Lu-PSMA cycles and 27% received ≥5 cycles2
aMeasured from start of 177Lu-PSMA therapy up to 90 days after last administration.
bMeasured from start of 177Lu-PSMA therapy up to 30 days after last administration.
Study Limitations: Chart review studies have bias related to treatment selection and unreported variables cannot be fully addressed. Outcomes are based on clinical judgment, with variability in patient and adherence that can result in different outcomes, therefore no conclusions can be drawn.2
Key Inclusion Criteria: Eligible patients were males aged ≥18 years, with bone metastases and a confirmed diagnosis of mCRPC, who had received ≥1 dose of XOFIGO and, in any subsequent therapy line, ≥1 dose of 177Lu-PSMA.2
REPORTED TEAEs AND LAB ABNORMALITIES DURING AND POST 177LU-PSMA TREATMENT2
REPORTED TEAEs2
Measured from the start of 177Lu-PSMA up to 30 days after last dose.
REPORTED GRADE 3-4 LABORATORY ABNORMALITIES2
AEs=adverse events; ASAT=aspartate aminotransferase; N=number of patients evaluated; n=number of patients with the specified event;
PSMA=prostate-specific membrane antigen; SAEs=serious adverse events; Tax=taxane-based chemotherapy; TEAE=treatment-emergent adverse event.
Measured from the start of 177Lu-PSMA up to 30 days after last dose.
References: 1. De Vincentis G, Gerritsen W, Gschwend JE, et al. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019;30(11):1728-1739. 2. Rahbar K, et al. Presented at European Society for Medical Oncology Annual Meeting; September 9-13, 2022; Paris, France.

By mimicking calcium, XOFIGO has a greater affinity for areas of increased bone turnover, such as metastases.1

XOFIGO emits alpha particles that induce double-strand DNA breaks. These hard-to-repair DNA breaks result in cell death within the tumor and microenvironment.1
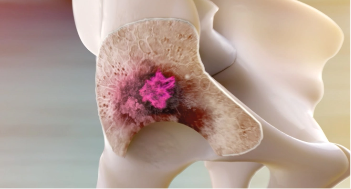
The short range of alpha particles emitted by XOFIGO (<10 cell diameters) limits damage to surrounding normal tissue.1
XOFIGO IS THE ONLY FDA-APPROVED TARGETED ALPHA THERAPY THAT TARGETS AND TREATS BONE METASTASES IN mCRPC1
USE XOFIGO TO TARGET OSTEOGENIC CELLS WHERE PROSTATE TUMORS PROLIFERATE.1
OSTEOGENIC CELLS SHOW THE HIGHEST ABSORPTION OF XOFIGO COMPARED WITH OTHER TISSUES1
The highest rate of absorption from the dosimetry data was recorded in osteogenic cells, which include osteoblasts where prostate cancer metastases can occur1
CALCULATED ABSORBED RADIATION DOSE TO ORGANS1,a
LLI=lower large intestine; MBq=megabecquerel; mGy=milligray; ULI=upper large intestine.
aCalculations of absorbed radiation doses were performed using OLINDA/EXM (Organ Level INternal Dose Assessment/EXponential Modeling). For radium-223, which is primarily an alpha particle-emitter, assumptions were made for intestine, red marrow, and bone/osteogenic cells to provide the best possible absorbed radiation dose calculations for XOFIGO, considering its observed biodistribution and specific characteristics. Additional particular modeling was applied for the lungs. The absorbed dose to the lungs is estimated as the dose contribution from Ra223 and daughter decays in the blood-containing fraction of the lung mass and also the dose contribution from 219Rn and daughter decays in the respiratory tract.
bColon dose = 0.57 X ULI dose + 0.43 X LLI dose.2
Reference: 1. Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019.
XOFIGO PATIENT CHECKLIST
PATIENTS WITH THE FOLLOWING MAY ALSO BE CANDIDATES FOR XOFIGO:
- Malignant lymph node involvment up to 3 cm1
- Patients referred for EBRT3
- Regardless of PSMA status in bone-predominant disease4
- Independent of taxane exposure; naïve or experienced1,5
- XOFIGO can be an option across multiple scanning modalities4:
- BONE
- CT
- PSMA-PET
References: 1. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. 2. Xofigo® (radium Ra 223 dichloride) injection [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2019. 3. Finkelstein SE et al. Presented at: 2015 Annual ASCO Meeting; May 29-June 2, 2015; Chicago, Illinois. Abstract 182. 4. Ahmadzadehfar H, Azgomi K, Hauser S, et al. Ga-PSMA-11 PET as a gatekeeper for the treatment of metastatic prostate cancer with 223Ra: proof of concept. J Nucl Med. 2017;58(3):438-444. 5. Sartor O, Hoskin P, Coleman RE, et al. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate. 2016;76(10):905-916.